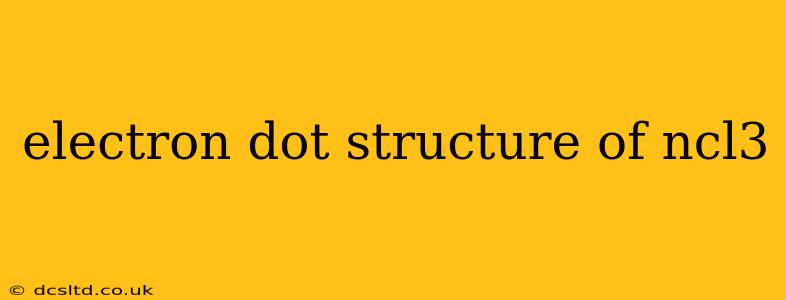
Nitrogen trichloride (NCl₃) is a fascinating inorganic compound with a unique electron dot structure. Understanding this structure is key to grasping its properties and reactivity. This guide will delve into the intricacies of NCl₃'s electron dot structure, answering common questions and providing a clear, step-by-step explanation.
What is the Electron Dot Structure of NCl₃?
The electron dot structure, also known as the Lewis structure, visually represents the valence electrons of atoms in a molecule. For NCl₃, nitrogen (N) is the central atom, surrounded by three chlorine (Cl) atoms. Nitrogen has 5 valence electrons, and each chlorine atom has 7. To achieve a stable octet (8 valence electrons), nitrogen shares one electron with each of the three chlorine atoms, forming three single covalent bonds. This leaves one lone pair of electrons on the nitrogen atom.
..
:Cl:
|
:N:
|
:Cl:
..
:Cl:
In this structure, each chlorine atom has a complete octet (two lone pairs and one bond pair), and nitrogen has a complete octet (one lone pair and three bond pairs).
How to Draw the Electron Dot Structure of NCl₃?
Drawing the Lewis structure is straightforward:
-
Count Valence Electrons: Nitrogen has 5, and each chlorine has 7, totaling 5 + (3 x 7) = 26 valence electrons.
-
Identify the Central Atom: Nitrogen, being less electronegative than chlorine, becomes the central atom.
-
Arrange Atoms: Place the three chlorine atoms around the central nitrogen atom.
-
Distribute Electrons: Place one electron pair between nitrogen and each chlorine to form single bonds. This uses 6 electrons (3 bonds x 2 electrons/bond).
-
Complete Octet Rule: Distribute the remaining 20 electrons (26 - 6 = 20) as lone pairs around the chlorine atoms, ensuring each chlorine atom has 8 electrons around it. The remaining lone pair is placed on the nitrogen atom.
-
Verify Octet Rule: Check that all atoms have a complete octet (except for potential exceptions like hydrogen).
What is the Molecular Geometry of NCl₃?
The molecular geometry of NCl₃ is trigonal pyramidal. This is because the presence of the lone pair on the nitrogen atom causes repulsion, pushing the three chlorine atoms slightly downward, resulting in a pyramid-like shape, not a planar structure.
What is the Hybridization of Nitrogen in NCl₃?
The hybridization of nitrogen in NCl₃ is sp³. This means one s orbital and three p orbitals of nitrogen hybridize to form four sp³ hybrid orbitals. Three of these orbitals overlap with the p orbitals of the chlorine atoms to form sigma bonds, while the fourth sp³ orbital contains the lone pair of electrons.
Is NCl₃ Polar or Nonpolar?
NCl₃ is a polar molecule. The trigonal pyramidal shape and the presence of a lone pair on the nitrogen atom create an uneven distribution of electron density, resulting in a net dipole moment.
What are the Properties of NCl₃?
Nitrogen trichloride is a highly reactive and unstable compound. It's a dense, oily liquid that is toxic and highly explosive, particularly in the presence of light or heat. Its instability is largely attributed to the relatively weak N-Cl bonds.
This comprehensive guide provides a detailed understanding of the electron dot structure of NCl₃, including its molecular geometry, hybridization, polarity, and properties. Remember, always handle NCl₃ with extreme caution due to its hazardous nature.