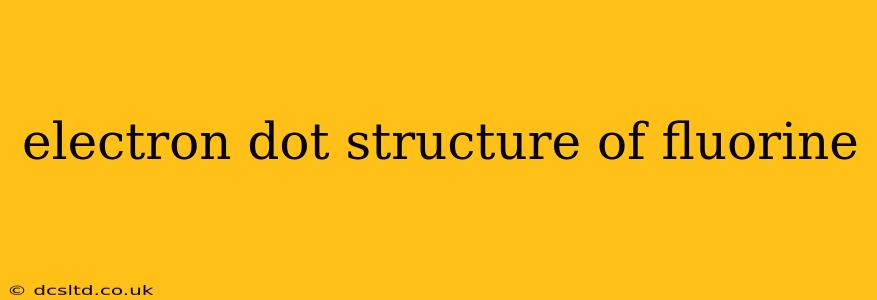
Fluorine, the most reactive element on the periodic table, boasts a simple yet fascinating electron dot structure. Understanding this structure is key to grasping its chemical behavior and reactivity. This guide will explore the electron dot structure of fluorine, answer common questions, and delve into its implications.
What is the Electron Dot Structure of Fluorine?
Fluorine (F) has an atomic number of 9, meaning it possesses 9 electrons. Its electron configuration is 1s²2s²2p⁵. In the electron dot structure, we only represent the valence electrons – the electrons in the outermost shell. For fluorine, these are the seven electrons in the 2s and 2p orbitals. Therefore, the electron dot structure of fluorine is represented as:
F•
or, more explicitly showing the 2s and 2p electrons, but still simplified:
•
F •••
•
This depiction shows the fluorine atom with seven valence electrons surrounding the element's symbol (F). This incomplete outer shell is the driving force behind fluorine's intense reactivity.
Why is Fluorine so Reactive?
H2. Why is the outer shell of fluorine incomplete?
Fluorine's high reactivity stems directly from its electron configuration. Atoms strive for a stable electron configuration, ideally resembling the noble gases with a full outer shell (octet rule). Fluorine, with only seven valence electrons, is one electron short of achieving a stable octet. This strong desire to gain that one electron makes it exceptionally reactive. It readily accepts an electron from other atoms to form a stable fluoride ion (F⁻).
H2. How does fluorine achieve a stable octet?
Fluorine achieves a stable octet by forming a single covalent bond with another atom. This bond involves sharing one electron with another atom, effectively completing its outer shell. For example, in a fluorine molecule (F₂), each fluorine atom shares one electron with the other, resulting in both atoms having eight valence electrons.
H2. What are some common compounds formed by fluorine?
Fluorine's high reactivity leads to the formation of numerous compounds. These include:
- Hydrogen fluoride (HF): A highly corrosive acid used in various industrial processes.
- Fluorocarbons: Compounds containing carbon and fluorine, used as refrigerants, solvents, and in various other applications. Some fluorocarbons are potent greenhouse gases.
- Fluorides: Salts containing the fluoride ion (F⁻), many of which are found in toothpaste to prevent tooth decay.
Implications of Fluorine's Electron Dot Structure
The electron dot structure of fluorine directly influences its chemical and physical properties. Its strong electronegativity (the ability to attract electrons) is a direct consequence of its incomplete octet. This makes fluorine exceptionally reactive, forming strong bonds with other elements.
Understanding fluorine's electron dot structure is crucial in various fields, including:
- Chemistry: Predicting reactivity, bonding behavior, and the formation of chemical compounds.
- Materials Science: Designing new materials with specific properties, such as high-strength polymers and superconductors.
- Medicine: Developing new drugs and therapies, understanding the role of fluoride in dental health.
In conclusion, the seemingly simple electron dot structure of fluorine holds the key to understanding its remarkable properties and its significant role in various aspects of science and technology. Its drive to complete its outer electron shell dictates its reactivity, making it a crucial element with widespread applications.