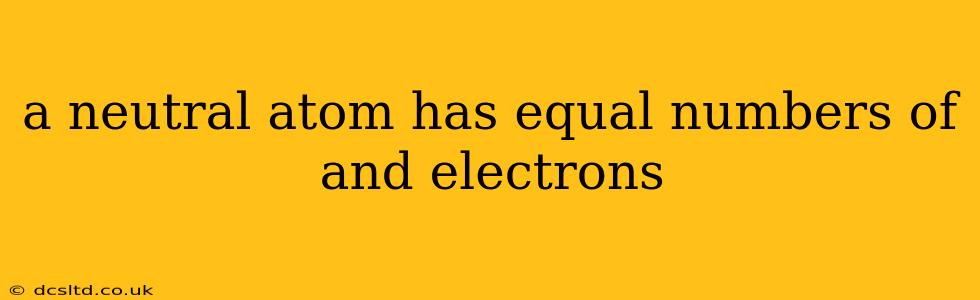
A Neutral Atom: Equal Protons and Electrons
A fundamental concept in chemistry and physics is the neutral atom. Understanding its composition is crucial to grasping the behavior of matter. Simply put, a neutral atom has an equal number of protons and electrons. This balance of positive and negative charges results in a net charge of zero.
Let's break this down further:
-
Protons: These are positively charged subatomic particles found in the nucleus of an atom. The number of protons defines the element; for example, all atoms with one proton are hydrogen, those with two are helium, and so on. This number is called the atomic number.
-
Electrons: These are negatively charged subatomic particles that orbit the nucleus in shells or energy levels. They are significantly lighter than protons and are responsible for chemical bonding and interactions.
-
Neutrons: While not directly involved in the neutrality of an atom, neutrons are also located in the nucleus and carry no charge (neutral). Their presence influences the atom's mass but not its overall charge.
Why is the equal number of protons and electrons important?
The equal number of protons and electrons is critical because it results in a balanced charge. The positive charge of the protons is exactly canceled out by the negative charge of the electrons. This balance is what makes the atom electrically neutral. If there's an imbalance – more protons than electrons, or vice versa – the atom becomes an ion, carrying a net positive (cation) or negative (anion) charge.
What happens if a neutral atom gains or loses electrons?
When a neutral atom gains or loses electrons, it no longer has an equal number of protons and electrons. This creates an ion:
-
Cation: A positively charged ion formed when an atom loses one or more electrons. The number of protons now exceeds the number of electrons.
-
Anion: A negatively charged ion formed when an atom gains one or more electrons. The number of electrons now exceeds the number of protons.
How can I determine the number of protons, electrons, and neutrons in an atom?
The number of protons is given by the atomic number of the element, found on the periodic table. In a neutral atom, the number of electrons is equal to the number of protons. The number of neutrons can be calculated by subtracting the atomic number from the mass number (the total number of protons and neutrons in the nucleus).
For example, consider Carbon (C):
- Atomic Number: 6 (This means it has 6 protons)
- Mass Number (usually a whole number close to the atomic weight): 12 (This is an approximation. Different isotopes of carbon have different mass numbers)
- Number of Electrons (in a neutral atom): 6 (equal to the number of protons)
- Number of Neutrons: 12 (mass number) - 6 (atomic number) = 6
What are isotopes?
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers. Because isotopes have the same number of protons and electrons, they remain electrically neutral. However, their different mass numbers lead to slight variations in their physical properties.
Understanding the equal number of protons and electrons in a neutral atom is fundamental to understanding atomic structure and chemical behavior. This knowledge forms the basis for exploring more complex concepts in chemistry and physics.